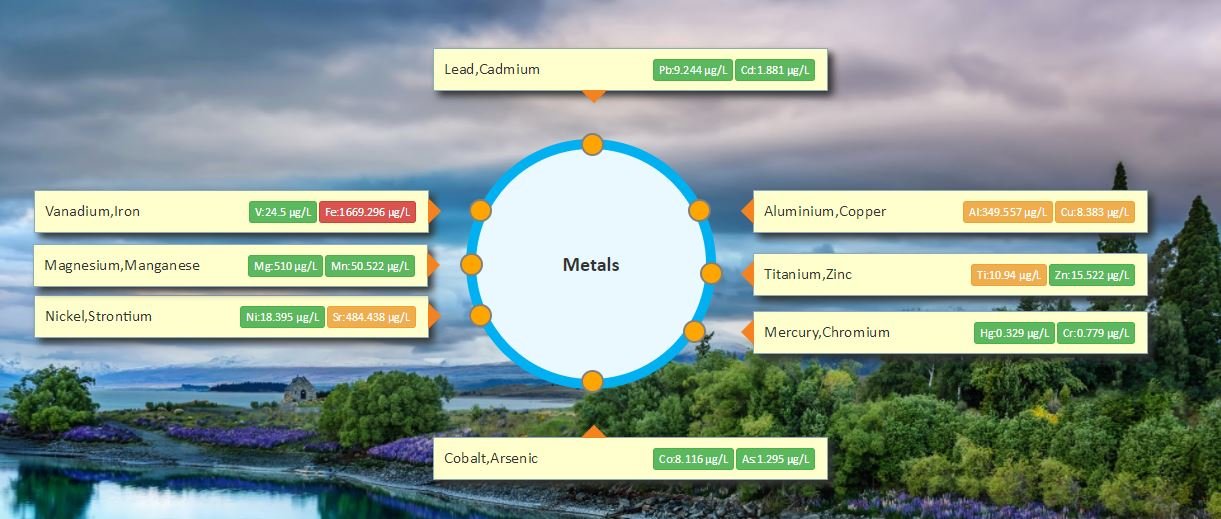
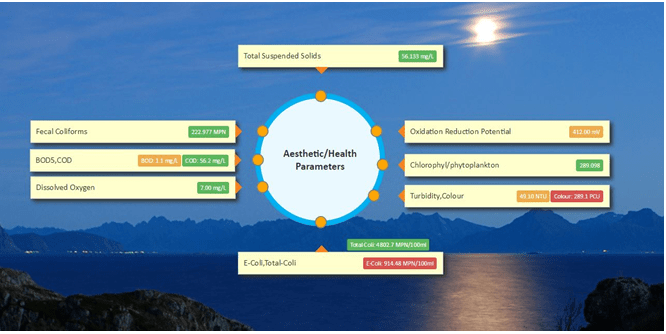
What are the parameters monitored using Water365?
- All parameters measured and estimated using AI engines are constantly compared with BIS-IS10500 parameters, for conformance, online via IoT for access via App and analytics.
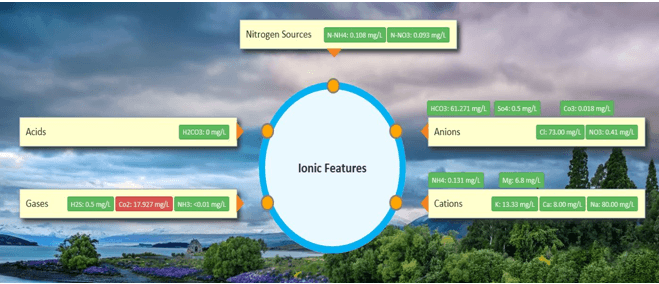
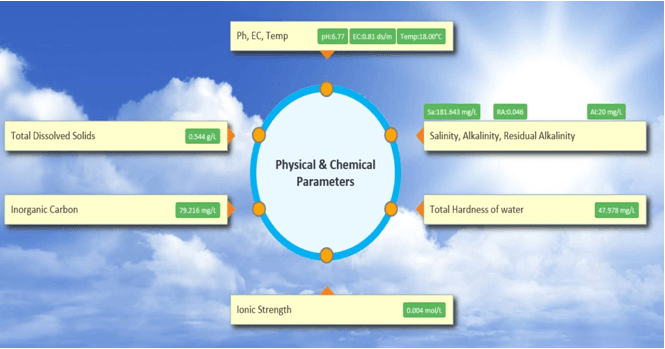
- Physical/Chemical: Ph, EC, TDS, TSS, Turbidity, DO, Water Temperature, Ammonia nitrogen (NH3-N), Total nitrogen, Total phosphorous
- Health/Aesthetic: E-Coli, Fecal Coliforms, Total Coliforms, BOD5, COD, Oxidation Reduction Potential (ORP)
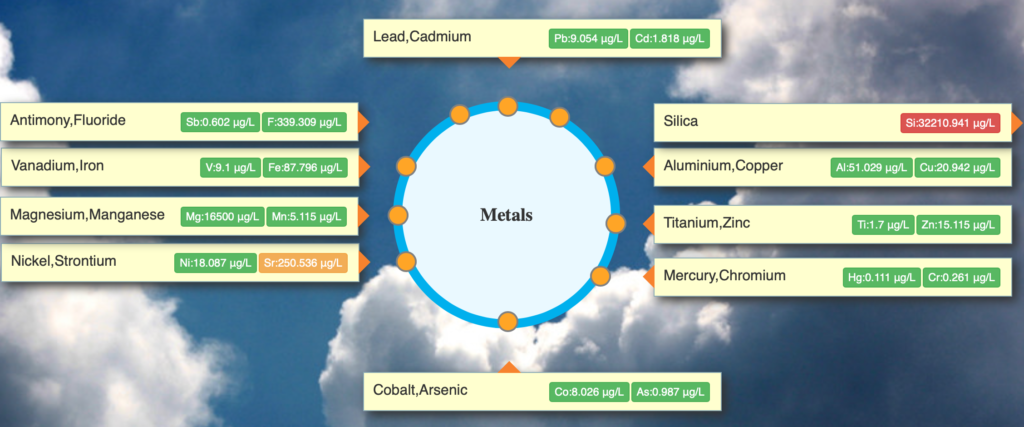
- Metals: Aluminium, Arsenic, Cadmium, Chromium, Cobalt, Copper, Cyanide, Flouride, Lead, Manganese, Mercury, Selenium, Nickel, Zinc
- To understand the dynamic condition of water body, four to six readings per day of all the water quality parameters are measured.
- The sensors installed are guaranteed for a year, after the installation, and are easily serviceable without excess maintenance.
- The sensors ideally should be high temperature resistant as the implementation of these will be in climates more than 40deg.C.
APP Driven analytics and on-line monitoring
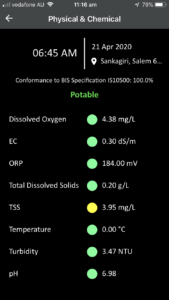


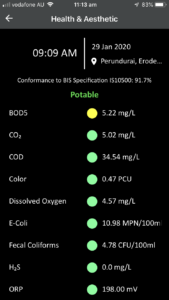
The web-based program with login credentials provides both a high level of security and personal access to data for organizations. Samples of web-based software output for various parameters are given below:

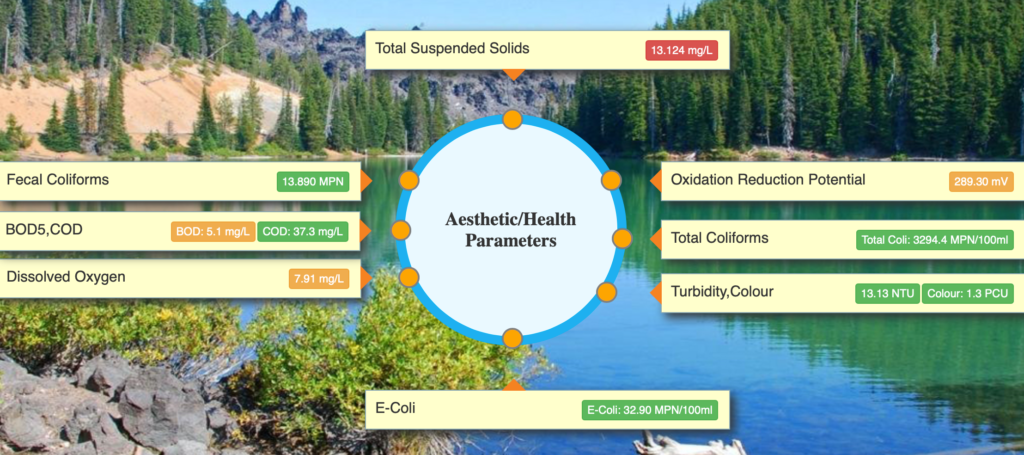
NOTES ON IMPORTANT WATER QUALITY PARAMETERS BOTH MEASURED/ESTIMATED
Sodium (Na+)
-
- Found in abundant rock-forming minerals typically occurring as colourless or pale-coloured crystals and consisting of aluminosilicates of potassium, sodium, and calcium.
- Sodium is second most abundant element dissolved in seawater.

- Sodium mobility is often influenced by humans: Use of water softeners, human or animal waste disposal, leachate from landfills are few examples of Sodium mobility in the environment.
- It is often associated with Chloride or Bromide.
- Can be toxic to plants in high concentrations.
Potassium (K+)
- Found in abundant rock-forming minerals typically occurring as colourless or pale-coloured crystals and consisting of aluminosilicates of potassium, sodium, and calcium.
- This is a key fertilizer component. The presence of Potassium is key for soil health, plant growth and animal nutrition.
- Activates enzymes involved in plant growth and plays a role in photosynthesis. Necessary for producing ATP in plants and plays a role in protein synthesis.
- It is often associated with Chloride or Bromide
Calcium Ca++
- Calcium is the most abundant of the alkaline-earth materials.
- This is derived from most of the rock-types like igneous, metamorphic and sedimentary rocks.
- Calcium is present in the form of Calcite (CaCO3) and gypsum salts (CaSO4-2H20) that are prevalent in sedimentary rocks.
- Calcium contributes to hardness of water along with Magnesium.
Chloride Cl-
- Chloride Cl- is a salt resulting from combining chlorine ions with metals like Na and Mg. Most of the salts formed like NaCl and MgCl2 are highly soluble in water.
- Chlorine as Cl2 is highly toxic and is used in paints, petroleum products, bleach, antiseptics, insecticides and solvents.
- Sources for Chloride is waste water from industries, agriculture runoffs, waste water treatment plant effluent and swimming pools run offs.
- This is toxic to coral and reef fish in high concentrations.
NO3-
- Enters water through nitrogen cycle rather than via dissolved minerals.
- In excessive amounts, it can lead to eutrophication. Eutrophication is excessive richness of nutrients in a lake or other body of water, frequently due to run-off from the land, which causes a dense growth of plant life.
- It is an important source of Nitrogen for animals and plants. Nitrogen from NO3 is represented as N-NO3.
- Excessive NO3 in human water consumption can be harmful and should be within the limits specified by the Australian Drinking Water Guidelines (ADWG)
HCO3-
- Bicarbonate is one component of alkalinity and its concentration is in a balance with carbon dioxide between the pH range of 4.4 and 8.2 and in a balance with carbonate between the pH range of 8.2 and 9.6.
CO3-
- Carbonate is one component of alkalinity and its concentration is in a balance with bicarbonate between the pH range of 8.2 and 9.6.
- At a pH of 9.6 and higher, there is no carbon dioxide or bicarbonate, with all alkalinity being in the carbonate form.
Mg++
- Magnesium is an important element for good health and a level around 10mg/L is desirable in drinking water. Very high levels of magnesium will produce scale and clog up water pipes.
SO42-
- Sulphate occurs naturally in surface waters and underground waters. The ADWG value for sulphate is 500mg/L and the aesthetic ADWG value is 250mg/L. Sulphate levels above 500mg/L can have purgative effects (upset stomach and vomiting).
- Levels <400 mg/L are considered acceptable for human consumption but levels above 250mg/L will cause aesthetic problems (appearance).
Ionic Strength (I)
- The ionic strength of a solution is a measure of the concentration of ions in that solution.
- Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation constant or the solubility of different salts. One of the m0ain characteristics of a solution with dissolved ions is the ionic strength.
- In this program, Ionic strength is calculated as “mol/L” and to avoid confusion the units should be stated explicitly.
EC
- Ionic concentrations in water are responsible for carrying an electrical current. Conductivity is related to the total dissolved solids and salinity of water.
- Rain water has a conductivity between 10 S/cm and 20 S/cm whilst sea water is around 50,000 S/cm. The conductivity of bore waters can vary widely from good quality (< 200 S/cm) to very saline (50,000 S/cm). (National Health and Medical Research Council 2004).
- EC depends on the water: the higher the temperature, the higher the electrical conductivity would be.
- High temperatures result in evaporation and loss of fresh water that will increase both conductivity and salinity of a waterbody. Warm weather can even increase ocean salinity.
EC25
- Ionic concentrations in water are responsible for carrying an electrical current.
- Conductivity is related to the total dissolved solids and salinity of water.
- EC 25 is a standardized reading of electrical conductivity at 25 deg. C.
- Rain water has a conductivity between 10 S/cm and 20 S/cm whilst sea water is around 50,000 S/cm. The conductivity of bore waters can vary widely from good quality (< 200 S/cm) to very saline (50,000 S/cm).
- EC depends on the water: the higher the temperature, the higher the electrical conductivity would be. EC 25 is a standardized measurement of EC at 25 deg.C. Most instruments nowadays automatically standardize the readings to 25 deg.C.
pH
- pH is a numeric scale used to specify the acidity or basicity of an aqueous solution. It is approximately the negative of the base 10 logarithms of the molar concentration, measured in units of moles per liter, of hydrogen ions.
- Solutions with pH less than 7 are acidic and solutions with pH greater than 7 are basic. Pure water is neutral, at pH, 7, being neither an acid or a base.
- Rainwater is always acidic (pH varies between about pH 4.5 to pH 6.5 since moisture (H2O) in the air absorbs carbon dioxide (CO2) producing carbonic acid (H2CO3).
TDS
- The total dissolved solids include silica plus cations plus anions but exclude bicarbonate because bicarbonate is lost when the dissolved material is dried. TDS is calculated as follows:
- TDS = SiO2 (mg/L) + Cations (mg/L) + Anions (mg/L) – (0.5083 x HCO3⎯)
- There is an empirical relationship between TDS and the Conductivity known as the I/C ratio. The “I” represents total dissolved solids minus silica in mg/L (I = TDS mg/L – Silica mg/L) and “C” is the conductivity in S/cm.
- I/C is calculated as follows and generally ranges in value from about 0.4 to 0.7 (average usually 0.5):
- I/C = (TDS – Silica),mg/L /Conductivity,S/cm≈ 0.6
Specific Gravity
- Specific gravity a measure of density. Determining the specific gravity of an object can tell you a lot about it, like whether it will float or sink in water. Usually specific gravity refers to an object’s density when compared with the density of water, so this value is a ratio.
- The specific gravity of a substance is calculated by dividing the specific gravity of that substance by the specific gravity of water. Thus, the specific gravity of pure water would be a number divided by itself, which will always equal one.
- The specific gravity of water depends on the type of water being measured. Pure water at 4 degrees Celsius has a specific gravity of one. If the water has salts in it, the water is denser and the specific gravity will be greater than one. Sea water, for instance, is denser than fresh or pure water. Water with a specific gravity of one has a weight of one gram per one milliliter.
Temperature
- Temperature is measured in C in Australia. Varying temperature has significant effect on the movement of ions, electrical conductivity, salinity and other reactants.
- Temperature plays a major role in solution stability.
Oxidation Reduction Potential (ORP)
- It is a measurement of water’s ability to oxidize contaminants. The higher the ORP, the greater the number of oxidizing agents. Checking ORP is a simple method to monitor the effectiveness of a sanitizer or the quantity of anti-oxidants in a liquid. In other words, it is a measure of tendency of chemical species in water to acquire electrons thereby “reduced”.
Dissolved Oxygen (DO)
- Oxygen dissolved in water can come from the atmosphere or as a by-product of the photosynthesis of aquatic plants.
- Water below the air/water interface is not necessarily in equilibrium with the air and can have even less oxygen than this small value.
- It is typical for the oxygen concentration in lakes and in the ocean to decrease with depth in the water.
- The dissolved oxygen levels are higher in the summer and during daylight hours because this is when photosynthetic organisms produce oxygen.
- Atmospheric pressure is lower at higher altitudes so water at higher elevations holds less dissolved oxygen than water at sea level.
Turbidity
- Turbidity occurs because of a suspension of fine colloidal particles that do not readily settle out of solution and can result in “cloudiness”.
- Turbidity is expressed in Nephelometric Turbidity Units (NTU) and is determined by an instrument known as a Nepholometer.
- Turbidity causes light to be scattered and absorbed rather than transmitted in straight lines through a sample and the Nepholometer measures the relative amount of light able to pass through the solution.
- There is no health-related guideline value for turbidity. However, for aesthetic reasons turbidity values should be <5 NTU. Waters with a turbidity <1 NTU can be safely disinfected without cause for concern for micro-organisms being protected by small particles. However, it is possible that waters with a turbidity >1
- NTU may shield some micro-organisms from disinfection.
CaCO3
- This mineral is usually found as a rock, like the stalactites and stalagmites in caves.
- It can also be dissolved in water and contributes to the hardness of water.
- It is also common in the form of calcite or a white mineral found in limestone and marbles.
- A white or colorlesscrystallinecompound,CaCO3, occurringnaturally in chalk,limestone,andmarble. It is used to maketoothpaste,whitepaint,andcleaningpowder.Total alkalinity is measured by collecting a water sample, and measuring the amount of acid needed to bring the sample to a pH of 4.2. At this pH all the alkaline compounds in the sample are “used up.” The result is reported as milligrams per liter (mg/l) of calcium carbonate.
Total Hardness
- Hardness in water is caused by the presence of high levels of calcium (Ca++) and magnesium (Mg++) ions. These ions combine with carbonate (CO32⎯) dissolved in water to produce scale which is composed of insoluble carbonates (CaCO3 and MgCO3). The scale builds up on plumbing parts, eventually causing blockages to water flow.
- Langelier Saturation Index (LSI) is a measure to determine the extent and risk of “scale build” up due to CaCO3. Refer to LSI for more explanation.
TSS
- It is the measure of total number of suspended or colloidal particles in water. It is measured in mg/L and although related to Turbidity, it should not be mistaken for Turbidity.
- Turbidity is measured by the amount of light which is reflected by the particles.
- The suspended or colloidal particles, commonly referred to as total suspended solids (TSS), are all the extremely small suspended solids in water which will not settle out by gravity.
Total Inorganic carbon
- Carbon present in solution mainly as carbonate and bicarbonate, but also cyanide and thiocyanate.
Alkalinity
- Alkalinity is due to bicarbonate (HCO3¯), carbonate (CO32¯) and hydroxide (OH¯) anions and is a measure of the water’s resistance (or buffering effect) to pH change when acid is added.
- This should not be mistaken for pH which is numeric scale to specify acidity or basicity of an aqueous solution.
Residual Alkalinity
- Residual Alkalinity (RA) is defined as the remainder of carbonate and bicarbonate after subtracting the equivalent of Ca++ and Mg++ i.e. if the equivalent concentrations of
HCO3¯ and CO32¯ ions exceed the concentrations of Ca++ and Mg++, the water is said to contain residual alkalinity. - RA is expressed as milli-equivalents per litre.
- Residual alkalinity serves as a commonly used index to determine the suitability of the water for irrigation purposes. It is calculated by subtracting the concentrations of calcium and magnesium from the concentrations of carbonate and bicarbonate expressed in milli-equivalents per litre (meq/L).
- RA = meq (HCO3¯ + CO3 2¯) – meq (Ca2+ + Mg2+)
- A high RA will be damaging to soil structure and crop growth because Ca and Mg will be precipitated from soil solutions increasing the relative concentration of Na
Salinity
- Firstly, this should not be confused with electrical conductivity.
- Salinity is a measure of the amount of salts in the water. Because dissolved ions increase salinity as well as conductivity, the two measures are related.
- Salts that dissolve in water break into positively and negatively charged ions. Conductivity is the ability of water to conduct an electrical current, and the dissolved ions are the conductors. The major positively charged ions are sodium, (Na+) calcium (Ca+2), potassium (K+) and magnesium (Mg+2). The major negatively charged ions are chloride (Cl-), sulfate (SO4-2), carbonate (CO3-2), and bicarbonate (HCO3-). Nitrates (NO3-2) and phosphates (PO4-3) are minor contributors to conductivity, although they are very important biologically.
- Because dissolved ions increase salinity as well as conductivity, the two measures are related.
Gases
H2CO3
- Carbonic acid (ancient name acid of air or aerial acid) is the only inorganic carbon acid, and has the formula H2CO3.
- It is also a name sometimes given to solutions of carbon dioxide in water, which contain small amounts of H2CO3.
- The salts of carbonic acids are called bicarbonates (or hydrogencarbonates) and carbonates.
CO2
- Carbon Dioxide is present in water in the form of a dissolved gas. Surface waters normally contain less than 10 ppm free carbon dioxide, while some ground waters may easily exceed that concentration.
- Carbon dioxide is readily soluble in water. Over the ordinary temperature range (0-30 C) the solubility is about 200 times that of oxygen.
- Calcium and magnesium combine with carbon dioxide to form carbonates and bicarbonates.
- Aquatic plant life depends upon carbon dioxide and bicarbonates in water for growth.
- Microscopic plant life suspended in the water, phytoplankton, as well as large rooted plants, utilize carbon dioxide in the photosynthesis of plant materials; starches, sugars, oils, proteins. The carbon in all these materials comes from the carbon dioxide in water.
H2S
- Hydrogen sulfide gas produces an offensive “rotten egg” or “sulfur water” odor and taste in the water.
- In some cases, the odor may be noticeable only when the water is initially turned on or when hot water is run. Heat forces the gas into the air which may cause the odor to be especially offensive in a shower. Occasionally, a hot water heater is a source of hydrogen sulfideodor.
- A nuisance associated with hydrogen sulfide includes its corrosiveness to metals such as iron, steel, copper and brass. It can tarnish silverware and discolor copper and brass utensils.
- Hydrogen sulfide also can cause yellow or black stains on kitchen and bathroom fixtures.
- The odor of water with as little as 0.5 ppm of hydrogen sulfide concentration is detectable by most people.
- Concentrations less than 1 ppm give the water a “musty” or “swampy” odor. A 1-2 ppm hydrogen sulfide concentration gives water a “rotten egg” odor and makes the water very corrosive to plumbing.
N-NO3
- Nitrogen as part of the NO3 is written as N-NO3.
- This Nitrogen is very important for plants and soil well being.
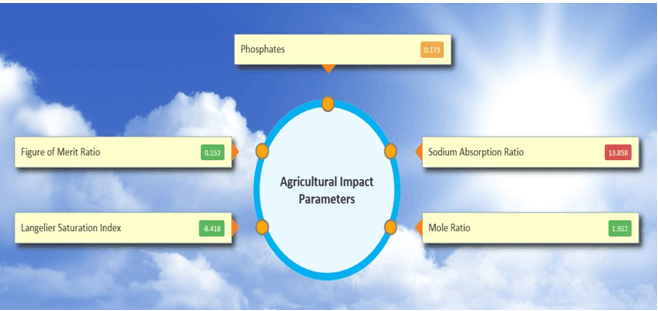
Sodium Absorption Ratio (SAR)
- The SAR is a parameter relevant to irrigation use and is a measure of the ratio of sodium to calcium and magnesium. When the ratio of sodium to calcium and magnesium is high, there is an adverse effect on soil structure and permeability, which can reduce crop yields and growth rates. Generally, the higher the SAR value, the more unsuitable is the water for low salt tolerant crops.
- SAR is calculated as follows: SAR = Na+/ (Ca+++ Mg++)/2
Mole Ratio
- The Mole Ratio is an index of corrosion. High values indicate high corrosive powers. If mole ratio >2, the water has high corrosivity.
Figure of Merit Ratio (FMR)
- The FM provides another indication for the suitability of the water for irrigation use. It is the ratio of the sum of Mg++ and Ca++ divided by Na+ expressed as meq/L. When comparing the formulae for FM and SAR, one is almost the reverse of the other. In this case however, the higher the FM value, the more suitable is the water for irrigation.
- Figure of Merit Ratio is calculated using: FM = (Ca+++ Mg++)/ Na+
Langelier Saturation Index
- The Langelier Saturation Index (LSI) is an approximate measure of the degree of saturation of water with calcium carbonate. If the LSI is <0, the water is under-saturated with respect to CaCO3 and therefore the water cannot form a protective coating of scale on plumbing parts.
- A reliable estimation of Langelier Saturation Index is very important as the value derived is used for estimating the effect of water on immersed parts.
Chlorophyl/phytoplankton
- Chlorophyll, in various forms, is bound within the living cells of algae and other phytoplankton found in surface water.
- Chlorophyll is a key biochemical component in the molecular apparatus that is responsible for photosynthesis, the critical process in which the energy from sunlight is used to produce life-sustaining oxygen.
- Chlorophyll is present in many organisms including algae and some species of bacteria. Chlorophylla is the most abundant form of chlorophyll within photosynthetic organisms and, for the most part, gives plants their green color.
- Chlorophyll is essential to the existence of phytoplankton.
- Phytoplankton can be used as an indicator organism for the health of a particular body of water. Monitoring chlorophyll levels is a direct way of tracking algal growth.
- Surface waters that have high chlorophyll conditions are typically high in nutrients, generally phosphorus and nitrogen.
Chemical Oxygen Demand (COD)
- Chemical Oxygen demand (COD) indicates the mass of Oxygen consumed per litre of solution.
- It is commonly used to indirectly measure the amount of organic compounds in water.
- Most applications of COD determine the amount of organic pollutants found in surface water (catchments, lakes and rivers), making COD a useful measure of water quality.
- COD is an index to assess the effect of discharged wastewater will have on the receiving environment.
- Higher COD levels mean a greater amount of oxidisable organic material in the lake, which will reduce dissolved oxygen (DO) levels.
- A reduction in DO can lead to anaerobic conditions, which is detrimental to higher aquatic life forms.
Escherichia Coli:
Escherichia coli is one of the most frequent causes of many common bacterial infections, including cholecystitis, bacteremia, cholangitis, urinary tract infection (UTI), and traveler’s diarrhea, and other clinical infections such as neonatal meningitis and pneumonia. The genus Escherichia is named after Theodor Escherich, who isolated the type species of the genus. Escherichia organisms are gram-negative bacilli that exist singly or in pairs. E coli is facultatively anaerobic with a type of metabolism that is both fermentative and respiratory. They are either nonmotile or motile by peritrichous flagella. E coli is a major facultative inhabitant of the large intestine.
E. coli is present in all mammalian feces at high concentrations; it does not multiply appreciably, but can survive in water for weeks, and so it is useful as an indicator of fecal pollution of drinking water systems. E. coli meets all the criteria used for the definition of both total coliforms and fecal coliforms. In addition, the organism can be distinguished from other fecal coliforms by the lack of urease and the presence of B-glucuronidase enzymes.
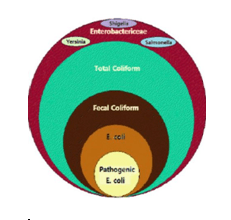
Fecal Coliform Bacteria
These organisms are a subset of the total coliform group. The fecal coliforms have the same properties as the coliform group, except that the fermentation is able to proceed at 44.5°–45.5°C. They are considered a better indicator of fecal contamination than the coliform group. Members of two bacteria groups, coliforms and fecal streptococci, are used as indicators of possible sewage contamination because they are commonly found in human and animal feces. Although they are generally not harmful themselves, they indicate the possible presence of pathogenic (disease-causing) bacteria, viruses, and protozoans that also live in human and animal digestive systems. Therefore, their presence in streams suggests that pathogenic microorganisms might also be present and that swimming and eating shellfish might be a health risk.
Color
“APHA” stands for American Public Health Association Color Scale, the organization responsible for the original definition and implementation of this visual color scale as a standard method for rating water quality. It is also called “Pt-Co” for Platinum-Cobalt Color as this visual color scale is based on stable liquid color standards made from chloroplatinate solutions. The scale ranges from distilled water at 0 (“water-white”) to a stock solution of 500 (parts per million of platinum cobalt to water). Intermediate Pt-Co color standards are made by dilution of the Pt-Co stock solution as described in ASTM D1209. Another name used for this same color scale is “Hazen”, named after Allen Hazen, the chemist who first defined the color scale for the American Public Health Association. When referenced as “Hazen Color”, the range is often above the typical 500 units associated with the APHA/Pt-Co, as in “1500 Hazen Color”. “APHA”, “PCU” and “Hazen” are three names for the same color scale.
Oxidation-Reduction Potential (ORP)
Oxidation-reduction potential (ORP) measures the ability of a lake or river to cleanse itself or break down waste products, such as contaminants and dead plants and animals. When the ORP value is high, there is lots of oxygen present in the water. This means that bacteria that decompose dead tissue and contaminants can work more efficiently. In general, the higher the ORP value, the healthier the lake or river is. However, even in healthy lakes and rivers, there is less oxygen (and therefore lower ORP values) as you get closer to the bottom sediments (mud; see the picture below of a lake bottom). This is because there are many bacteria working hard in the sediments to decompose dead tissue, and they use up a lot of the available oxygen. In fact, oxygen disappears very quickly in the bottom mud (often within a centimeter or two) and ORP falls quickly. ORP is measured in addition to dissolved oxygen because ORP can provide scientists with additional information of the water quality and degree of pollution, if present. Also, there are other elements that can function like oxygen (in terms of chemistry) and contribute to increased ORP.
Biological Oxygen Demand
Biochemical oxygen demand is the amount of dissolved oxygen needed by aerobic biological organisms to break down organic material present in a given water sample at certain temperature over a specific time period. The BOD value is most commonly expressed in milligrams of oxygen consumed per litre of sample during 5 days of incubation at 20 °C and is often used as a surrogate of the degree of organic pollution of water.
 Contact: Vishy Karri
Contact: Vishy Karri
Email: expert365au@gmail.com
For more details of this technology, including pricing, contact Expert365 team.